报告时间:2017年4月20日(星期四)15:00-16:00
报告地点:能源基础楼一楼会议室
报告人:Dr. Shengfa Ye
Max-Planck Institute for Chemical Energy Conversion
报告摘要:
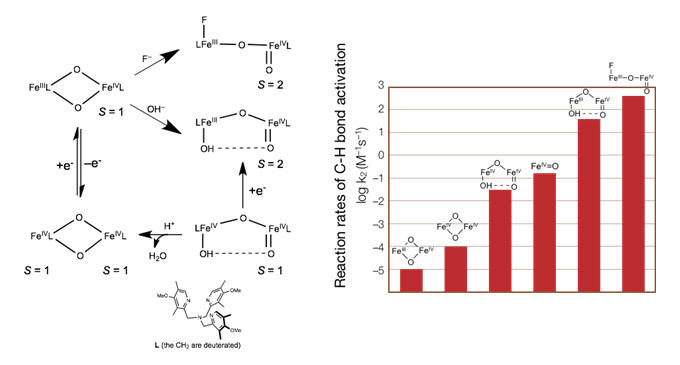
High-valent iron-oxo species have been postulated as key intermediates in various pivotal biological and chemical processes. Recently, Que and coworkers reported differential reactivity of a series of mono- and di-nuclear and iron(IV)-oxo complexes towards C-H bond activation, which reflect the effects of the core geometries (open vs. diamond core), the spin state of the reactive ferryl site (S = 1 vs. 2) and hydrogen bonding. To rationalize the observed diverse reactivity, we first analyze their electronic structures in detailby using a combined spectroscopic and computational approach, because the electronic structure and reactivity are intimately linked. Then we correlate the electronic structures with the reactivity. Our results reveal that in the reaction the diamond-core complexes undergo higher degree of the electronic and geometric readjustments than the open-core complexes, viz. the reorganization energies dictate their different reactivity. More importantly, the quintet iron(IV)-oxo species is more potent in activatingC-H bond than the triplet conger, because different reaction channels are employed.
报告人简介:
Education & Work Experiences
2011-present Staff scientist (group leader) at the Max-Planck Institute for Chemical Energy Conversion (Previously the Max-Planck Institute for Bioinorganic Chemistry), Mülheim (Ruhr), Germany
2009-2011 Group leader at the Institute of Physical and Theoretical Chemistry, University of Bonn, Germany
2006-2009 Post-doctor in the group of Prof. Dr. F. Neese at the Institute of Physical and Theoretical Chemistry, University of Bonn, Germany
2005-2006 Post-doctor in the group of Profs. Drs. K. Wieghardt and F. Neese at the Max-Planck Institute for Bioinorganic Chemistry, Mülheim (Ruhr), Germany
2001-2005 Doctoral work under guidance of Prof. Dr. W. Kaim at the Institute of Inorganic Chemistry, University of Stuttgart, Germany
Title: Electronic and structural properties of transition metal complexes with reactive N, S, Omixed-donor ligands
Research Work
General Interest: Elucidation of electronic structures of transition metal complexes and rationalization of the reaction mechanisms thereof.
Computational Chemistry
 Bioinorganic chemistry(Structure, spectroscopy and reactivity of transition metal centers in enzymes)
Bioinorganic chemistry(Structure, spectroscopy and reactivity of transition metal centers in enzymes)
 Oxygen activation by non-heme iron enzymes
Oxygen activation by non-heme iron enzymes
 C-H bond activation by high-valent iron intermediates(FeIV,FeV,FeVI)
C-H bond activation by high-valent iron intermediates(FeIV,FeV,FeVI)
 Biological transformation of NO
Biological transformation of NO
 Copper enzymes
Copper enzymes
 Coordination chemistry
Coordination chemistry
 Transition metals with coordinated radicals (geometric and electronic structure, spectroscopy and reactivity)
Transition metals with coordinated radicals (geometric and electronic structure, spectroscopy and reactivity)
 Multiplets and optical spectra of transition metal complexes
Multiplets and optical spectra of transition metal complexes
 Molecular Magnetism
Molecular Magnetism
 CO2 fixation by transition metal complexes
CO2 fixation by transition metal complexes
Quantum Chemistry and Theoretical Spectroscopy
 Theory of M?ssbauer spectroscopy
Theory of M?ssbauer spectroscopy
 Theory of molecular magnetism
Theory of molecular magnetism
 Theory of optical spectroscopy (UV/vis, CD, MCD, resonance Raman)
Theory of optical spectroscopy (UV/vis, CD, MCD, resonance Raman)
Experimental Interests
 Optical Spectroscopy: Resonance Raman, CD, MCD, Absorption
Optical Spectroscopy: Resonance Raman, CD, MCD, Absorption
 Magnetic Spectroscopy: EPR, MCD, M?ssbauer
Magnetic Spectroscopy: EPR, MCD, M?ssbauer
报告联系人:505组 方堃(9307)

